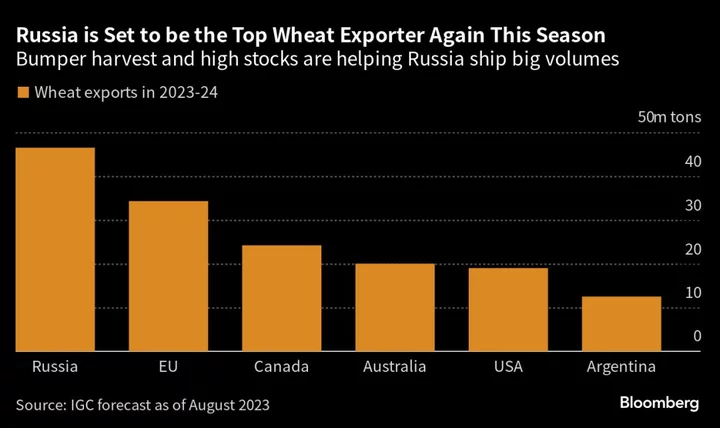
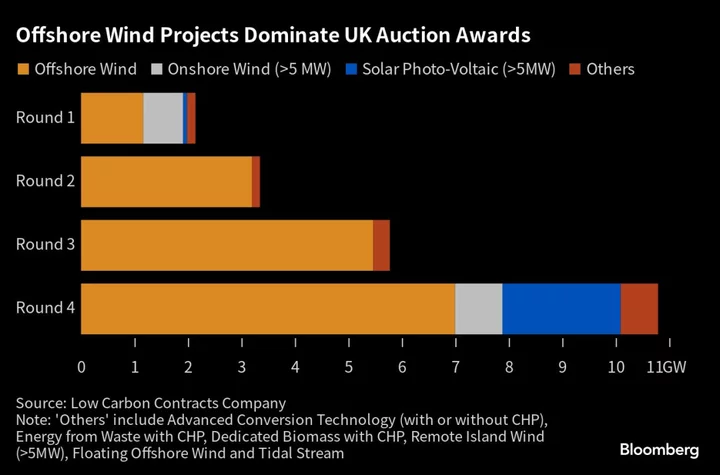
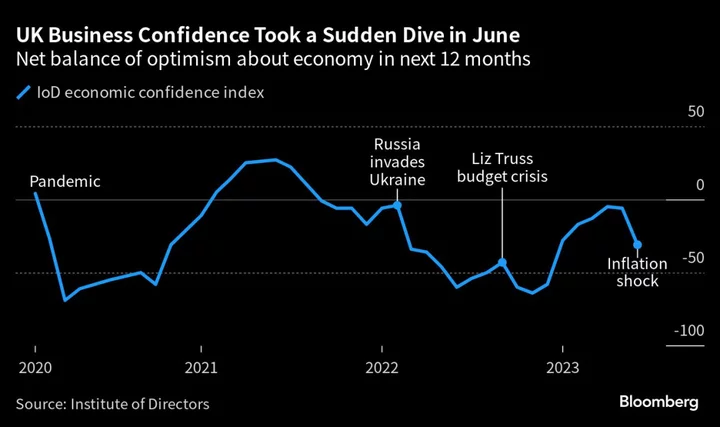
The Biden administration asked the US Supreme Court to uphold broad access to a widely used abortion pill, seeking a review of a ruling that would bar mail-order prescriptions and require in-person doctor visits.
The appeal, along with a similar petition filed Friday by the drug’s manufacturer, could draw the high court back into one of the nation’s most bitterly contested and politically potent issues, setting up a ruling next year in the middle of the presidential campaign.
Under its normal schedule, the court could say by the end of the year whether it will hear the case. It would be the court’s first full review in an abortion case since 2022 when it overturned the landmark Roe v. Wade opinion.
The justices in April issued an emergency order keeping mifepristone, as the pill is known, fully available while a lawsuit pressed by anti-abortion organizations and doctors went forward. The court gave no explanation for that order, though two justices publicly dissented.
A US appeals court last month backed new limits on mifepristone. The 2-1 ruling by the New Orleans-based 5th US Circuit Court of Appeals partially upheld a federal trial judge’s decision that would have effectively banned the sale of the drug.
The 5th Circuit said Food and Drug Administration decisions made starting in 2016 were likely unlawful and should be rolled back. The panel said the FDA failed to adequately address safety concerns.
Those changes included an extension of mifepristone’s approval to the 10th week of pregnancy — three weeks longer than was the case previously.
US Solicitor General Elizabeth Prelogar said in court papers that the 5th Circuit had imposed “novel requirements that have no basis” in federal law.
“FDA’s actions were supported by an exhaustive review of a record including dozens of scientific studies and decades of safe use of mifepristone by millions of women in the United States and around the world,” argued Prelogar, the Biden administration’s top Supreme Court lawyer.
Pill-based abortion has become the most popular method for terminating a pregnancy in the US and has emerged as a key target for anti-abortion advocates in the aftermath of last year’s ruling. Mifepristone is used as part of a two-pill regimen to end pregnancies and treat miscarriages. It is followed by misoprostol, which can also be used on its own to terminate a pregnancy.
“For the women and teenage girls, health care providers, and states that depend on FDA’s actions to ensure safe and effective reproductive health care is available, this case matters tremendously,” the drug’s manufacturer, Danco Laboratories LLC, said in its appeal. “And for the pharmaceutical and biotechnology industry, permitting judicial second-guessing of FDA’s scientific evaluations of data will have a wildly destabilizing effect.”
The lawsuit is being pressed by Alliance Defending Freedom, a Christian legal advocacy group that has been involved in many of the court’s highest-profile cases in recent years.
“By repeatedly and unlawfully removing critical safeguards in the chemical abortion regimen, the FDA has failed to protect the safety of women and girls,” ADF Senior Counsel Erik Baptist said in an emailed statement. “Two courts have now held the FDA accountable for the damage it has done to the rule of law and the harm it has caused to countless girls and women.”
--With assistance from Madlin Mekelburg and Fiona Rutherford.