LOS ANGELES--(BUSINESS WIRE)--Aug 24, 2023--
ChromaDex Corp. (NASDAQ:CDXC), a global authority on Nicotinamide Adenine Dinucleotide (NAD + ) research and healthy aging, shares findings from a preclinical study, as reported in the peer-reviewed journal International Journal of Molecular Sciences by a team of scientists led by Dr. Yue Yang, Assistant Professor of Research in Pharmacology at Weill Cornell Medicine in New York.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230824959294/en/
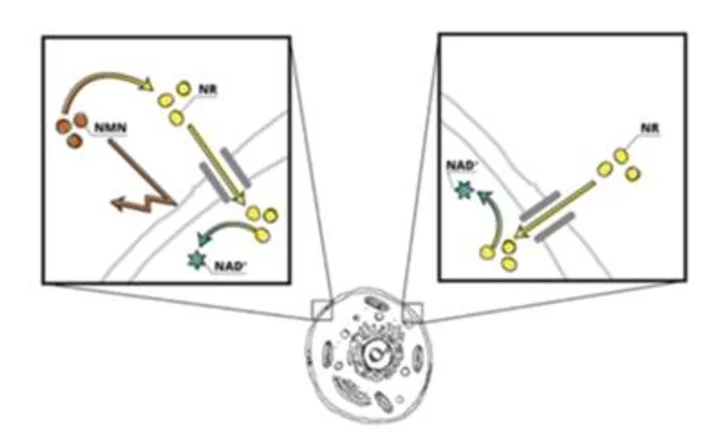
Outside of the cell, NMN cannot cross the cell barrier directly and must first be converted to NR (Graphic: Business Wire)
This preclinical study utilized stable isotopes, the gold standard method in research, to assess the metabolic fate of nicotinamide mononucleotide (NMN). The results indicate that NMN is primarily converted to nicotinamide and nicotinamide riboside (NR) before being utilized for NAD + synthesis, with only a small portion of NMN being directly incorporated into NAD +. These results build on a growing body of research demonstrating that NMN cannot cross the cell membrane directly and must first be converted to NR. Because NR can cross the cell membrane directly, this data, along with other science, indicates that NR is a more efficient NAD + precursor than NMN.
“The research overwhelmingly demonstrates that NMN obtained from the diet or supplementation is a precursor to NR as NMN cannot efficiently cross the cell membrane and suggests NR is more efficient at increasing NAD + levels,” said Dr. Anthony Covarrubias, Principal Investigator at UCLA David Geffen School of Medicine, Department of Microbiology, Immunology, and Molecular Genetics, UCLA Metabolism and Immunology Theme Space, who was not involved in the study.
Although NAD + was discovered over a century ago, interest in the science around NAD + biology has ignited due to the recent discovery of NR and NMN’s ability to boost bioavailable NAD +. However, in November 2022, the U.S. Food & Drug Administration determined that NMN could no longer be sold as a dietary supplement. This decision has no impact on NR, which is a chemically and biologically distinct precursor to NAD +.
Chemical structure differences of NR and NMN
NR and NMN are chemically identical except for a phosphate group present within the structure of NMN. This phosphate group prevents exogenous NMN from entering the cell directly. NMN’s phosphate group is removed by the extracellular enzyme, CD73, converting NMN into NR. A comparison of two separate clinical studies featuring healthy middle-aged to older adults who received 1000 mg of NR or NMN daily showcased that NR was 25% more effective in raising whole blood NAD + levels after two weeks of supplementation. Additionally, because of the additional phosphate on NMN, an individual must consume approximately 15% more NMN (~345mg), compared to 300mg of NR to deliver the same amount of NAD + precursor molecules ( Conze et al., 2019 and Pencina et al., 2022 ).
While one published preclinical study claimed the NMN transporter, Slc12a8, allows NMN to enter cells directly, these results were obtained in mice and never replicated in humans ( Grozio et al., 2019 ). By comparison, five preclinical studies have demonstrated that NMN cannot cross the cell barrier directly and must first be converted to NR to increase NAD + ( Fletcher et al., 2017, Nikiforov et al., 2011, Ratajczak et al., 2016, Kim et al., 2020, Suave et al., 2023 ). In contrast, NR is directly imported into human cells by a family of equilibrative nucleoside transporter (ENT) proteins ( Kropotov e al., 2021 ).
About the study
The preclinical study led by Dr. Yang provides insights into NAD + homeostasis and the precise effects of NMN in mice. 10-week-old male mice were administered isotopically-labeled NMN (500 mg/kg) both intravenously and orally and NAD + synthesis was analyzed after 2 or 4 hours.
Study highlights
- The liver had the highest increase in NAD + levels after NMN administration, but it actively broke down NMN releasing it in the blood, and preferred utilizing nicotinamide and NR instead of NMN for NAD + production
- The kidney and small intestines showed significant incorporation of NR for NAD + production from NMN
- There was a very small amount of direct incorporation of NMN into NAD + in the kidney and white adipose tissue, however levels were nominal
The results indicate that NMN is primarily converted to nicotinamide and NR before powering the crucial synthesis of NAD + and only a small portion of NMN was directly incorporated into NAD +. This understanding of the conversion pathways sheds light on the dynamics of NAD + metabolism in different tissues and is consistent with a large body of evidence suggesting NMN must be converted into NR prior to entering cells.
Further, another recent preclinical study analyzing NR and NMN demonstrated that while both NMN and NR protect DNA, there was a stronger preventative effect against DNA damage when cells were treated with NR before chemotherapy versus NMN ( Qiu et al., 2023 ).
These results add to the growing body of science establishing that NR is a more efficient and superior NAD + precursor compared to NMN, however more research must be conducted to determine the effects of NR and NMN supplementation on the full NAD + metabolome.
For additional information on ChromaDex, visit www.chromadex.com.
About ChromaDex:
ChromaDex Corp. is a global bioscience company dedicated to healthy aging. The ChromaDex team, which includes world-renowned scientists, is pioneering research on nicotinamide adenine dinucleotide (NAD + ), levels of which decline with age. ChromaDex is the innovator behind NAD + precursor nicotinamide riboside (NR), commercialized as the flagship ingredient Niagen®. Nicotinamide riboside and other NAD + precursors are protected by ChromaDex’s patent portfolio. ChromaDex maintains a website at www.chromadex.com to which ChromaDex regularly posts copies of its press releases as well as additional and financial information about the Company.
Forward-Looking Statements:
This release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities and Exchange Act of 1934, as amended, including statements related to whether this research builds on a growing body of evidence showcasing that exogenous NMN must be converted to NR, making NR a more efficient NAD + precursor to NMN. Statements that are not a description of historical facts constitute forward-looking statements and may often, but not always, be identified by the use of such words as "expects," "anticipates," "intends," "estimates," "plans," "potential," "possible," "probable," "believes," "seeks," "may," "will," "should," "could" or the negative of such terms or other similar expressions. Risks that contribute to the uncertain nature of these forward-looking statements include the impact of the COVID-19 pandemic on our business and the global economy; our history of operating losses and need to obtain additional financing; the growth and profitability of our product sales; our ability to maintain sales, marketing and distribution capabilities; changing consumer perceptions of our products; our reliance on a single or limited number of third-party suppliers; and the risks and uncertainties associated with our business and financial condition. More detailed information about ChromaDex and the risk factors that may affect the realization of forward-looking statements is set forth in ChromaDex's Annual Report on Form 10-K for the fiscal year ended December 31, 2022, ChromaDex's Quarterly Reports on Form 10-Q and other filings submitted by ChromaDex to the SEC, copies of which may be obtained from the SEC's website at www.sec.gov. Readers are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof, and actual results may differ materially from those suggested by these forward-looking statements. All forward-looking statements are qualified in their entirety by this cautionary statement and ChromaDex undertakes no obligation to revise or update this release to reflect events or circumstances after the date hereof.
View source version on businesswire.com:https://www.businesswire.com/news/home/20230824959294/en/
CONTACT: ChromaDex Media Contact:
Kendall Knysch, Head of Media Relations & Partnerships
310-388-6706 ext. 689
kendall.knysch@chromadex.comChromaDex Investor Relations Contact:
+1 (949) 356-1620
InvestorRelations@ChromaDex.com
KEYWORD: CALIFORNIA UNITED STATES NORTH AMERICA
INDUSTRY KEYWORD: HEALTH GENETICS RESEARCH PHARMACEUTICAL SCIENCE BIOTECHNOLOGY
SOURCE: ChromaDex Corporation
Copyright Business Wire 2023.
PUB: 08/24/2023 08:32 AM/DISC: 08/24/2023 08:32 AM
http://www.businesswire.com/news/home/20230824959294/en