GENEVA--(BUSINESS WIRE)--Sep 4, 2023--
Medimaps Group, a Swiss/Global med-tech company specializing in image-processing software with AI capabilities for assessing bone health, announced today it received the Medical Device Regulation (MDR) certification from its notified body BSI (CE 2797) for its management system and product portfolio.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230904380765/en/
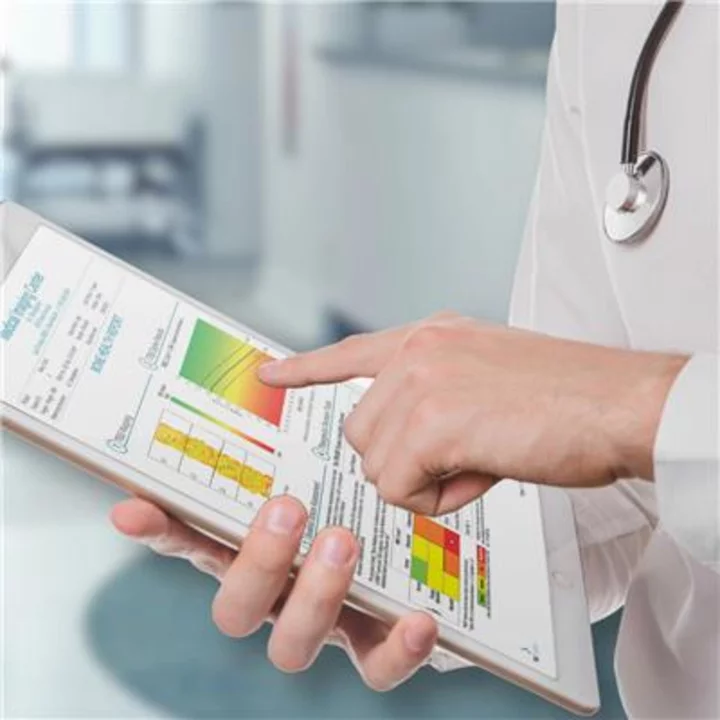
Medimaps Group TBS iNsight™ software assesses patients' bone microarchitecture to help detect fracture risk (Photo: Medimaps Group)
“We are very proud to be one of the earliest AI companies to achieve the MDR certification. By successfully navigating the rigorous requirements of the Medical Device Regulation, our company proves its ability to deliver innovative and reliable solutions that meet the highest regulatory standards for the benefit of patients, healthcare professionals and medical business partners”, said Prof. Didier Hans, co-founder and CEO of Medimaps Group.
“The MDR framework is of utmost importance as it governs our processes to develop, manufacture, market, and monitor the safety of our products. This is the result of a two-year effort to execute this complex regulatory process, which guarantees the highest manufacturing standards and traceability for our medical software”, added Meinhard F. Schmidt, chairman of the board of Directors of Medimaps Group.
The new MDR came into effect in Europe in May 2021 and it represents the most significant change to the European healthcare regulatory framework in decades. The certification ensures that medical device manufacturers meet the most stringent quality requirements in order to commercialize their products in Europe. It upgrades the 93/42/EEC directive certification which the company has held for years, confirming its solidity and reliability to deliver high quality products. Through the MDR, Medimaps Group was able to increase the robustness of its clinical evaluation, technical design, and post market surveillance.
With this certification, Medimaps Group can continue to supply without interruptions certified TBS iNsight™ software at the highest standard to its valued customers in Europe and subsequent markets. TBS iNsight™ is a medical image processing device seamlessly integrated into dual-energy X-ray absorptiometry (DXA) scanners, and/or X-rays and CT PACs systems, used to measure BMD to detect bone fragility in osteoporosis patients. The software provides deep tech bone texture analysis related to bone micro-architecture and complements BMD measuring and clinical risk factors to refine the management of osteoporosis without further examination or radiation.
TBS iNsight™ is recommended in over 30 medical guidelines worldwide and published in over 1,000 peer-reviewed scientific articles. It is CE marked, FDA approved enabling commercial availability in 60 countries worldwide. It benefits from reimbursement in certain countries, including dedicated CPT codes in the USA.
About Medimaps Group
Medimaps Group is a Swiss-based company developing and marketing software as a medical device solutions. Our medical imaging software applications are developed with the patient in mind, and are based on patented multi-purpose technology with artificial intelligence capabilities, providing healthcare solutions that fit seamlessly into the workflow. Our lead product TBS iNsight™ (Osteo) has been used for years in clinical practice worldwide in the field of osteoporosis, and is established as the gold standard for bone texture assessment in clinical practice. Learn more at https://www.medimapsgroup.com.
View source version on businesswire.com:https://www.businesswire.com/news/home/20230904380765/en/
CONTACT: Saoyuth Nidh
Global Corporate Communications Manager
Mob: +41 79 969 77 57
snidh@medimapsgroup.com
KEYWORD: EUROPE SWITZERLAND UNITED STATES NORTH AMERICA
INDUSTRY KEYWORD: TECHNOLOGY MEDICAL DEVICES OTHER TECHNOLOGY SOFTWARE BIOTECHNOLOGY OTHER HEALTH HEALTH GENERAL HEALTH ARTIFICIAL INTELLIGENCE
SOURCE: Medimaps Group
Copyright Business Wire 2023.
PUB: 09/04/2023 09:02 AM/DISC: 09/04/2023 09:02 AM
http://www.businesswire.com/news/home/20230904380765/en