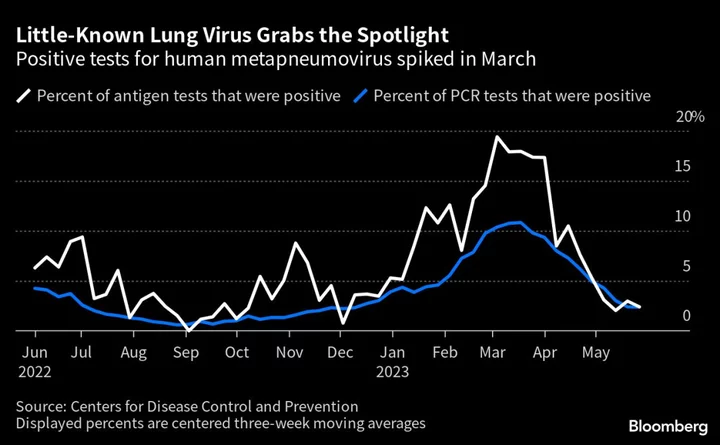
A little-known respiratory virus is grabbing the limelight from Covid-19 and RSV after cases surged earlier this year, spurring companies to prepare their vaccines for a waiting market.
About one in five US lung patients who were tested in March for the illness, called human metapneumovirus, showed signs of the disease, according to the Centers for Disease Control and Prevention. That uptick mirrors the post-pandemic spread of other viruses like influenza that abated during lockdowns and resurged as Covid prevention measures lapsed.
The virus, a relatively obscure cousin of respiratory syncytial virus, can cause infections like bronchitis or pneumonia that can be severe in children and older adults, according to the CDC. At least five companies are developing treatments and vaccines, some in combination with shots against more-familiar RSV, but none have yet progressed to the late-stage trials that usually precede market entry.
The disease’s activity has returned to normal patterns, CDC spokesperson Scott Pauley said in an email. Companies are persisting with their efforts to develop effective prevention in anticipation of future outbreaks.
Seattle-based Icosavax Inc. announced positive early human trial results last week for a combination RSV/human metapneumovirus vaccine for older adults. Both viruses have been shown to significantly contribute to viral pneumonia in adults and children, and few treatments are available. The company plans to initiate a mid-stage trial later this year and select a formulation to move forward with next year, Chief Executive Officer Adam Simpson said.
“We believe that with hMPV, we would be able to potentially double the protection against viral pneumonia that older adults experience” compared to only having an RSV vaccine, Simpson said in an interview. The recent CDC data show that human metapneumovirus treatment is a “major unmet need,” he said.
Moderna Inc., one of the key players in Covid and RSV vaccines, is recruiting subjects for a trial of a similar combination shot, and some participants have already received doses, according to a May securities filing. Massachusetts-based AlloVir Inc. is conducting an early- to mid-stage clinical trial for a treatment of respiratory tract infections including human metapneumovirus in high-risk patients.
Other companies list the disease as a key development target. Valneva SE has completed laboratory work on a vaccine and is exploring partnerships to develop a combination shot. The virus is the “second largest medical need in the respiratory area” and this year’s peak in cases “makes us even more determined to find the right partner to progress our hMPV vaccine candidate as soon as we can,” Valneva Chief Executive Officer Thomas Lingelbach said in an email.
Enanta Pharmaceuticals Inc, working to develop an oral antiviral treatment, said it expects to select a combination RSV/human metapneumovirus candidate in the last three months of the year. The recent increase in cases doesn’t change the company’s plans, but provides “an additional sense of urgency for our program and helps bring awareness to the virus,” Enanta Chief Executive Officer Jay Luly said in an email.
AlloVir fell 1.9% in trading before US markets opened and Moderna edged up 0.3%. Enanta and Icosavax were unchanged. Valneva shares gained 1.1% in Paris.
Pandemic lockdowns halted the spread of respiratory viruses that typically spread during the winter and spring seasons, and companies have increasingly focused on corresponding vaccines since then. Human metapneumovirus activity in the US had remained low from March 2020 through May 2021, according to the CDC.
(Corrects premarket share prices in 10th paragraph.)