LOS ANGELES--(BUSINESS WIRE)--Jul 27, 2023--
Cutting-edge neuromodulation research being conducted by the SpineX team is once again being recognized by the world’s leading scientists, with the publication this month of two more peer-reviewed publications, both in Frontiers.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230727598285/en/
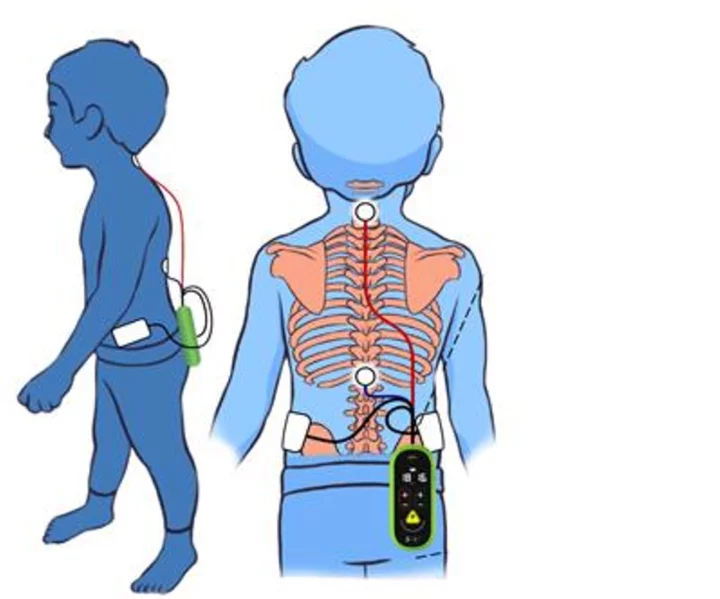
SpineX technology has been proven to treat the root cause of CP through spinal cord neuromodulation. (Graphic: Business Wire)
Frontiers is a leading journal in its field, publishing rigorously peer-reviewed research in rehabilitation from biological, clinical, and socio-humanistic perspectives. Its publications investigate new ways to optimize the functioning and wellbeing of people who experience disability, focusing on rehabilitation as the health strategy of the 21st century.
“Having our research validated by our peers and published in Frontiers is an honor, and also a testament to the innovation driving this work,” says SpineX CEO Parag Gad. “Thanks to this research, we can now confidently say that consistent, ongoing noninvasive spinal cord neuromodulation results in demonstrable improvement of sensorimotor function in children with Cerebral Palsy.”
Cerebral Palsy (CP) is the most common of all childhood disabilities, with about 500,000 children and over 250,000 adults in the United States living with the disease. It’s caused by abnormal neural connectivity between the brain and spinal cord, and affects motor function, impeding the ability to move voluntarily, and causing inability or difficulty walking and other day-to-day challenges.
SpineX is applying its novel, patented neuromodulation technology that acts as a hearing aid for the spine; amplifying the correct neural signals in order to help the patient better control muscle movement. The company has been conducting clinical trials of its non-invasive therapy platforms which have the potential to dramatically improve the lives of children living with CP. As demonstrated in recent publications, the company’s proprietary SCiP™ device targets the abnormal connectivity between brain and spinal cord, thereby treating the root causes of CP. SpineX plans on conducting a multisite trial starting in 2024 following which it will approach the US FDA for regulatory approval.
“Our experience with SCiP has been a true game-changer for our child,” says Jessica and Van Oldham, whose nine-year old daughter Annika lives with CP. “When she started her first series of therapy sessions, we saw almost immediate results. She went from using a walker or crutches almost exclusively to standing up straighter and using just one crutch/walking stick. She has also begun to walk independently in our home.”
Annika received SCiP over two near-consecutive therapy sessions, one of 16 weeks and the other of 10 weeks duration. The therapy is non-invasive and pain-free, making it ideal for treating children and adults.
In addition to advancing SCIP therapy for CP, SpineX has pioneered SCONE™ therapy. An experimental medical device that treats urinary incontinence in adults, SCONE allows people living with neurogenic bladder due to spinal cord injury, multiple sclerosis or stroke to live their lives on their own terms. Neuromodulation continues to bring dramatic, tangible results for patients afflicted with different debilitating chronic illnesses.
“As parents of a child with CP it’s a bit like fitting together a puzzle,” says Annika’s mother, Jess. “This is a very large piece of the puzzle; we saw more positive change in our daughter over the two courses of SCiP™ therapy than we did in the first seven years of her life. She says she feels more aligned and is literally taking bigger steps all the time.”
About SpineX Inc.
SpineX Inc., a clinical stage bioelectric MedTech company developing Noninvasive Spinal Neuromodulation devices as a platform technology. SCONE™ and SCiP™ are two FDA-designated Breakthrough Devices being developed by SpineX for the treatment of adults with Neurogenic Bladder and Children with Cerebral Palsy respectively. To learn more about SpineX, visit www.spinex.co or follow @spinex_inc on social media.
View source version on businesswire.com:https://www.businesswire.com/news/home/20230727598285/en/
CONTACT: Parag Gad, CEO
media@spinex.co
KEYWORD: CALIFORNIA UNITED STATES NORTH AMERICA
INDUSTRY KEYWORD: RESEARCH GENERAL HEALTH PHARMACEUTICAL CONSUMER MEDICAL DEVICES HOSPITALS HEALTH TECHNOLOGY CHILDREN SCIENCE SURGERY BIOTECHNOLOGY HEALTH
SOURCE: SpineX Inc.
Copyright Business Wire 2023.
PUB: 07/27/2023 10:45 AM/DISC: 07/27/2023 10:46 AM
http://www.businesswire.com/news/home/20230727598285/en