LAKE ZURICH, Ill.--(BUSINESS WIRE)--Oct 9, 2023--
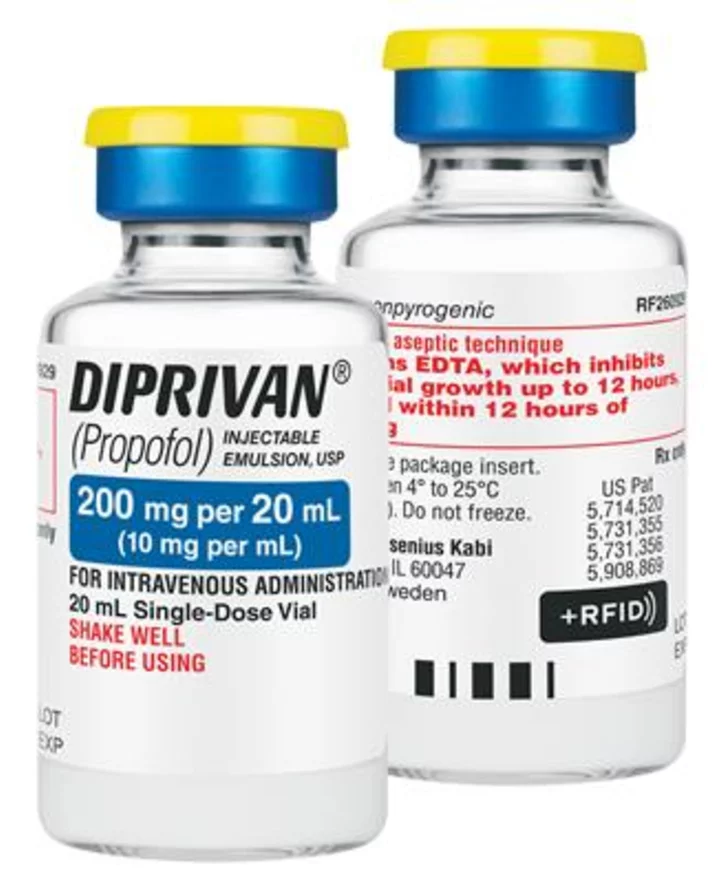
Fresenius Kabi announced today it has introduced +RFID smart labels for Diprivan ® (Propofol) Injectable Emulsion, USP, 200 mg per 20 mL in single-dose vials, sold in the United States. The +RFID labels are now fully compatible with all major RFID kit and tray systems in the U.S.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20231009314483/en/
Fresenius Kabi Diprivan® (Propofol) Injectable Emulsion, USP, 200 mg per 20 mL in single-dose vials, now compatible with all major RFID kit and tray systems. (Photo: Business Wire)
The launch expands Fresenius Kabi’s industry-leading +RFID portfolio of fully interoperable labeled medications. The use of radio-frequency identification (RFID) label technology eliminates the need to tag medicines manually which saves time and supports a safe, more efficient medication inventory process.
Fresenius Kabi is the first pharmaceutical manufacturer to adopt GS1's EPC Tag Data Standard (TDS) to enhance interoperability. GS1 Standards for identifying, capturing and sharing supply chain data help ensure product and inventory information is accessible, accurate and easy to understand.
Diprivan, the most prescribed propofol anesthetic in the U.S. 1, was the first Fresenius Kabi product introduced with RFID labels in 2020 for use with some RFID kit and tray systems. The drug is now certified for use with all RFID systems and is available immediately to order through wholesalers and direct from Fresenius Kabi.
To meet the highest standards of performance and quality, in addition to the company’s own testing, Diprivan +RFID has been tested by the Axia Institute of Michigan State University and certified by the ARC RFID lab of Auburn University. Fresenius Kabi is expanding its portfolio of fully compatible +RFID products based on demand from hospitals. In a recent study by the ASHP Foundation, nearly nine in 10 hospitals that participated reported they are already using RFID technology or are evaluating it.
“We are dedicated to supporting our customers in the adoption of RFID technology that enhances patient care, improves efficiency and streamlines workflows for pharmacists,” said John Ducker, president and CEO of Fresenius Kabi USA.
The company has also released newly labeled Vasopressin Injection, USP 20 units per 1 mL in single-dose vials with +RFID smart labels, adding to the company’s growing portfolio of products compatible with all major RFID kit and tray systems.
About Diprivan ® (Propofol), Injectable Emulsion, USP
Indications and Usage – DIPRIVAN ® (Propofol), Injectable Emulsion, USP is an intravenous general anesthetic and sedation drug for use in adults for initiation and maintenance of Monitored Anesthesia Care (MAC) sedation, combined sedation and regional anesthesia, and Intensive Care Unit (ICU) sedation of intubated, mechanically ventilated patients. DIPRIVAN is also indicated for induction of general anesthesia for patients greater than or equal to 3 years of age and for maintenance of general anesthesia for patients greater than or equal to 2 months of age.
Important Safety Information
DIPRIVAN is contraindicated in patients with a known hypersensitivity to propofol or any of DIPRIVAN components, and also in patients with allergies to eggs, egg products, soybeans or soy products.
DIPRIVAN is not indicated for use in pediatric patients for ICU sedation or for MAC sedation for surgical, nonsurgical or diagnostic procedures as safety and effectiveness have not been established.
DIPRIVAN is not recommended for induction of anesthesia in patients below the age of 3 years or for maintenance of anesthesia in patients below the age of 2 months because its safety and effectiveness have not been established in those populations.
Use of DIPRIVAN has been associated with both fatal and life-threatening anaphylactic and anaphylactoid reactions. Clinical features of anaphylaxis, including angioedema, bronchospasm, erythema, and hypotension occur rarely following DIPRIVAN administration.
Strict aseptic technique must always be maintained during handling. DIPRIVAN is a single access parenteral product (single patient infusion vial) which contains 0.005% disodium edetate (EDTA) to inhibit the rate of growth of microorganisms, for up to 12 hours, in the event of accidental extrinsic contamination. However, DIPRIVAN can still support the growth of microorganisms, as it is not an antimicrobially preserved product under USP standards. Failure to use aseptic technique when handling DIPRIVAN was associated with microbial contamination of the product and with fever, infection, sepsis, other life-threatening illness, and death. Do not use if contamination is suspected. Discard unused drug product as directed within the required time limits. There have been reports, in the literature and other public sources, of the transmission of bloodborne pathogens (such as Hepatitis B, Hepatitis C, and HIV) from unsafe injection practices, and use of propofol vials intended for single use on multiple persons. A DIPRIVAN vial is never to be accessed more than once or used on more than one person.
DIPRIVAN has no vagolytic activity. Reports of bradycardia, asystole, and rarely, cardiac arrest have been associated with DIPRIVAN. Pediatric patients are susceptible to this effect, particularly when fentanyl is given concomitantly.
Diprivan should not be coadministered through the same IV catheter with blood or plasma because compatibility has not been established.
For general anesthesia or monitored anesthesia care (MAC) sedation, DIPRIVAN should be administered only by persons trained in the administration of general anesthesia and not involved in the conduct of the surgical/diagnostic procedure. Sedated patients should be continuously monitored, and facilities for maintenance of a patent airway, providing artificial ventilation, administering supplemental oxygen, and instituting cardiovascular resuscitation must be immediately available. Patients should be continuously monitored for early signs of hypotension, apnea, airway obstruction, and/or oxygen desaturation. These cardiorespiratory effects are more likely to occur following rapid bolus administration, especially in the elderly, debilitated, or ASA-PS III or IV patients.
Administer DIPRIVAN with caution in patients with disorders of lipid metabolism such as primary hyperlipoproteinemia, diabetic hyperlipemia, and pancreatitis.
Very rarely the use of DIPRIVAN may be associated with the development of a period of postoperative unconsciousness which may be accompanied by an increase in muscle tone.
When DIPRIVAN is administered to an epileptic patient, there is a risk of seizure during the recovery phase.
Attention should be paid to minimize pain on administration of Diprivan.
Venous sequelae, i.e., phlebitis or thrombosis, have been reported rarely (less than 1%).
Perioperative myoclonia, rarely including convulsions and opisthotonos, has occurred in association with DIPRIVAN administration.
Rarely, cases of unexplained postoperative pancreatitis (requiring hospital admission) have been reported after anesthesia in which DIPRIVAN was one of the induction agents used.
For sedation of intubated, mechanically ventilated patients in the Intensive Care Unit (ICU), DIPRIVAN should be administered only by persons skilled in the management of critically ill patients and trained in cardiovascular resuscitation and airway management. Patients should be continuously monitored for early signs of significant hypotension and/or cardiovascular depression, which may be profound.
Abrupt discontinuation of DIPRIVAN prior to weaning or for daily evaluation of sedation levels should be avoided. This may result in rapid awakening with associated anxiety, agitation, and resistance to mechanical ventilation. In pediatric patients, abrupt discontinuation of DIPRIVAN following prolonged infusion may result in flushing of the hands and feet, agitation, tremulousness and hyperirritability. Increased incidences of bradycardia, agitation, and jitteriness have also been observed.
For both adult and pediatric ICU sedation, DIPRIVAN infusion has been associated with Propofol Infusion Syndrome, that have resulted in death. The syndrome is characterized by severe metabolic acidosis, hyperkalemia, lipemia, rhabdomyolysis, hepatomegaly, renal failure, ECG changes and/or cardiac failure.
Since DIPRIVAN is formulated in an oil-in-water emulsion, elevations in serum triglycerides may occur when DIPRIVAN is administered for extended periods of time. Patients at risk of hyperlipidemia should be monitored for increases in serum triglycerides or serum turbidity.
EDTA is a strong chelator of trace metals – including zinc. DIPRIVAN should not be infused for longer than 5 days without providing a drug holiday to safely replace estimated or measured urine zinc losses. At high doses (2 grams to 3 grams per day), EDTA has been reported, on rare occasions, to be toxic to the renal tubules. Monitor patients at risk for renal impairment.
There have been rare reports of pulmonary edema in temporal relationship to the administration of DIPRIVAN, although a causal relationship is unknown.
Neurosurgical Anesthesia: When DIPRIVAN is used in patients with increased intracranial pressure or impaired cerebral circulation, significant decreases in mean arterial pressure should be avoided because of the resultant decrease in cerebral perfusion pressure.
Cardiac Anesthesia: Slower rates of administration should be utilized in premedicated patients, geriatric patients, patients with recent fluid shifts, and patients who are hemodynamically unstable.
Pediatric Neurotoxicity: Published animal studies demonstrate that the administration of anesthetic and sedation drugs that block NMDA receptors and/or potentiate GABA activity increase neuronal apoptosis in the developing brain and result in long-term cognitive deficits when used for longer than 3 hours. The clinical significance of these findings is unclear.
Adverse reactions reported with an incidence greater than 1% were: bradycardia, arrhythmia, tachycardia, hypotension, decreased cardiac output, hypertension, movement, apnea, respiratory acidosis during weaning, rash, pruritus, burning/stinging or pain at injection site, and hyperlipemia.
To report SUSPECTED ADVERSE REACTIONS, contact Fresenius Kabi USA, LLC at 1-800-551-7176, option 5, or FDA at 1-800-FDA-1088 orwww.fda.gov/medwatch.
DIPRIVAN is not recommended for obstetrics, including Cesarean section deliveries. DIPRIVAN crosses the placenta, and as with other general anesthetic agents, the administration of DIPRIVAN may be associated with neonatal depression.
DIPRIVAN is not recommended for use in nursing mothers because Propofol has been reported to be excreted in human milk, and the effects of oral absorption of small amounts of propofol are not known.
This Important Safety Information does not include all the information needed to use DIPRIVAN® (Propofol), Injectable Emulsion, USP safely and effectively. Please see full prescribing information for DIPRIVAN® (Propofol), Injectable Emulsion, USP at www.fresenius-kabi.com/us.
References
1. Data on file.
About Fresenius Kabi
Fresenius Kabi ( www.fresenius-kabi.com/us ) is a global health care company that specializes in injectable medicines, biosimilars, and technologies for infusion, transfusion, and clinical nutrition. The company’s products and services are used to help care for patients with critical and chronic conditions. The company’s U.S. headquarters is in Lake Zurich, Illinois. The company’s global headquarters is in Bad Homburg, Germany. To learn about U.S. career opportunities at Fresenius Kabi, visit us at www.fresenius-kabi.com/us/join-us and follow us on LinkedIn.
View source version on businesswire.com:https://www.businesswire.com/news/home/20231009314483/en/
CONTACT: Joanie Clougherty (614) 717-5741
joan.clougherty@fresenius-kabi.com
KEYWORD: UNITED STATES NORTH AMERICA ILLINOIS
INDUSTRY KEYWORD: DATA MANAGEMENT TECHNOLOGY MANUFACTURING HEALTH GENERAL HEALTH PHARMACEUTICAL TRANSPORT HEALTH TECHNOLOGY SOFTWARE LOGISTICS/SUPPLY CHAIN MANAGEMENT PACKAGING
SOURCE: Fresenius Kabi
Copyright Business Wire 2023.
PUB: 10/09/2023 08:00 AM/DISC: 10/09/2023 08:03 AM
http://www.businesswire.com/news/home/20231009314483/en