European biotech firm Argenx SE is scheduled to release key drug trial data this summer. Deal-hungry Big Pharma is closely watching.
Several major drugmakers keen to expand in immunology have been studying the $23 billion company for some time and have it at the top of their wish lists, according to people familiar with the matter. Argenx has been working with JPMorgan Chase & Co. to help identify options in the event of any takeover bid, the people said, asking not to be identified because the information is private.
Interest is expected to pick up if results in July show that a key Argenx drug—already approved for one autoimmune disorder—can effectively tackle another disease, the people said. The therapy, called Vyvgart, could eventually generate up to $10 billion in peak sales if approved for all the conditions it’s being studied for, according to analysts at Robert W. Baird & Co.
“This could be a mega blockbuster drug,” said Yaron Werber, a senior biotechnology analyst at TD Cowen. “It’s going to be too hard for strategic acquirers to ignore this opportunity. It’s too lucrative, too unique, too profitable, too strategic.”
‘Best in Class’
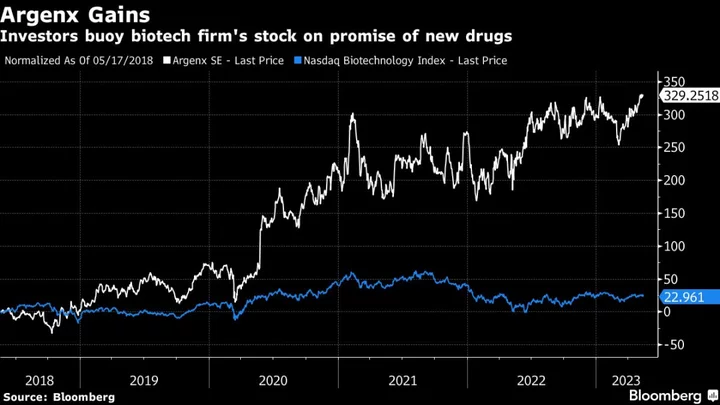
Argenx shares rose as much as 5.8% in Brussels trading on Wednesday, their biggest intraday jump since November 2022. The stock was up 3.7% at 9:50 a.m. in the Belgian capital, giving the company a market value of about €21.4 billion ($23.2 billion). The company’s American depositary shares have gained about 31% in New York trading over the past 12 months, outpacing the 15% gain in the Nasdaq Biotechnology Index.
The company has designed a new class of drug designed to block and remove certain harmful antibodies from the blood, targeting diseases where a person’s immune system attacks their own body. The technology makes Argenx a tempting target for companies including Roche Holding AG, Novartis AG, Eli Lilly & Co., AstraZeneca Plc, Merck & Co. and AbbVie Inc., Werber said.
Argenx will release data from a pivotal trial in July that will show whether Vyvgart is an effective treatment for chronic inflammatory demyelinating polyneuropathy, or CIDP, where nerve damage can impair patients’ ability to walk. Success could trigger hopes that the drug, already approved for generalized myasthenia gravis, might be applied to a wide range of other conditions with few or no compelling treatments.
“Having a drug that’s first in class, best in class, but on top of that having indications that are reasonably large—you don’t get those opportunities every day,” said David Nierengarten, a managing director on Wedbush Securities Inc.’s research team. “I think that does increase the likelihood of it getting bought.”
There’s always a possibility that Argenx could opt to start engaging with suitors before the release of the topline CIDP data expected in July, some of the people said. Still, the company hasn’t decided on a potential sale, and its already lofty valuation may deter some potential bidders, they said.
“We are focused on continuing to create value for patients and our shareholders,” a spokesperson for Argenx said, declining to comment on any potential deals. “We are well-positioned and remain committed to continuing to do that independently.”
Representatives for JPMorgan, AstraZeneca, Eli Lilly, Merck, Novartis and Roche declined to comment. A spokesperson for AbbVie didn’t immediately respond to a request for comment.
Pharma companies have been increasingly interested in treatments for autoimmune and inflammatory diseases because one drug can often be used for many different applications. In April, Merck agreed to buy autoimmune drug developer Prometheus Biosciences Inc. for about $10.8 billion.
High Price
Potential acquirers studying Argenx would want to wait for the July data from the CIDP trial before moving ahead to avoid any embarassment, said Joel Beatty, a senior research analyst at Baird. A positive result could bring in an additional $1.3 billion of peak sales for Argenx, he said.
“It would make it a lot easier for large pharma or other biotech companies to pay a relatively high price,” Beatty said.
Argenx is developing treatments for a host of diseases ranging from immune thrombocytopenia, a chronic bleeding disorder, to pemphigus vulgaris, a severe condition that causes blisters on the skin and in the mouth.
It’s led by co-founder Tim Van Hauwermeiren, a bioengineering specialist who previously worked as a business development executive at Ablynx NV and Procter & Gamble Co. The company takes its name from the Argonauts, the band of heroes in ancient Greek mythology who teamed up to find the Golden Fleece.
Argenx, which also trades in Brussels, has previously formed partnerships or done licensing deals with drugmakers including AbbVie, Eli Lilly, LEO Pharma A/S, Shire Plc and Boehringer Ingelheim GmbH.
To be sure, recent moves by US regulators could have a chilling effect on what until now has been a robust time for health care dealmaking.
The Federal Trade Commission sued Tuesday to block Amgen Inc.’s $27.8 billion acquisition of Horizon Therapeutics Plc, arguing the tie-up would stifle competition for the development of treatments for serious illnesses. The move created alarm that the government will upend big drugmakers’ standard playbook of snapping up smaller companies to build up their pipelines.
The other key question will be whether any potential acquirer can convince Argenx to do a deal. Though the founder doesn’t have a large enough stake to block a bid, the board could opt to wait for an even higher price before welcoming overtures.
“The stock is going to get a lot more expensive and will require a big premium. And we don’t believe this management team or board are interested sellers,” TD Cowen’s Werber said. “I think they’re interested in building a great company on their own.”
--With assistance from Suzi Ring, Naomi Kresge, Nacha Cattan and Angelica Peebles.
(Adds shares in fifth paragraph.)
Author: Dinesh Nair and Michelle F. Davis