MUNICH--(BUSINESS WIRE)--Jul 20, 2023--
CatalYm today announced the publication of preclinical data in Nature Communications under the title “ Tumor-derived GDF-15 blocks LFA-1 dependent T cell recruitment and suppresses responses to anti-PD-1 treatment ”. The study reveals a central role of GDF-15 in the resistance of tumors to current immunotherapy. These findings further highlight the therapeutic significance of CatalYm’s proprietary anti-GDF-15 antibody candidate, visugromab, currently in advanced Phase 2 clinical studies.
This press release features multimedia. View the full release here: https://www.businesswire.com/news/home/20230720231828/en/
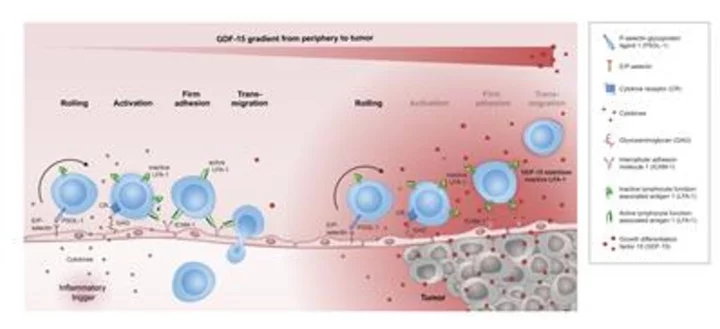
Action of GDF-15 as illustrated by CatalYm, all rights reserved. (Graphic: Business Wire)
This foundational research, which was performed together with founder Jörg Wischhusen and collaborators, is the first to describe a mechanistic link between tumor-produced Growth Differentiation Factor-15 (GDF-15) and the LFA-1/ICAM-1 cell adhesion axis. The interaction between LFA-1/ICAM-1 is a critical step in the infiltration of T cells into the tumor microenvironment. This is essential for the extravasation of T cells from the blood vessels into the surrounding tissue. GDF-15 is primarily known for its function in feto-maternal tolerance, an immunosuppressive mechanism that protects the fetus from the mother’s immune system.
The researchers demonstrated that GDF-15 blocks LFA-1-dependent T cell recruitment into the tumor microenvironment, a prerequisite for responses to anti-PD-1/-L1 treatment but also other immunotherapeutic strategies. Conversely, the blockade of GDF-15 with antibodies like the anti-GDF-15 antibody visugromab improved T cell infiltration into tumors. Combined with a PD-1 inhibitor, it increased tumor clearance and survival with a synergistic effect. In line with these findings, serum analysis of melanoma patients showed that response to anti-PD-1 negatively correlates with GDF-15 serum levels. Therefore, GDF-15 serum levels may be a predictive biomarker for the response to anti-PD-1 therapy and overall survival in these patients. These findings further underscore the immunosuppressive role of GDF-15 in the tumor and contribute to the growing body of data supporting GDF-15-neutralizing therapy as a promising approach for hard-to-treat tumors resistant or refractory to anti-PD-1/-L1 treatment.
"Our publication is the first to demonstrate the effect of tumor-derived GDF-15 on the LFA-1/ICAM-1 axis. As this axis orchestrates cell-cell-interactions that are essential for immune-mediated tumor control, GDF-15 likely contributes to immune escape across many different tumors and therapies,” said Prof. Dr. Jörg Wischhusen, Co-Founder of CatalYm and Professor at the Julius-Maximilians-University Würzburg, who is the senior author of the publication.
“Unraveling the details of the underlying biologic pathways is crucial to develop effective therapeutic approaches that can reverse tumor-mediated immunosuppression resulting in drug resistance, one of the major challenges in cancer medicine. We are committed to rapidly advancing our Phase 2 evaluation of visugromab on our mission to expand the treatment horizon for current and future immunotherapies,” added Dr. Christine Schuberth-Wagner, Chief Scientific Officer at CatalYm.
The published data adds further valuable mechanistic understanding to the clinical findings from CalaYm’s GDFather ( GDF -15 A ntibody-media T ed H uman E ffector Cell R elocation) trials with visugromab in combination with the anti-PD-1 inhibitor nivolumab in patients with advanced solid tumors. The Phase 1 ( NCT04725474 ) study results announced in September 2022 showed an excellent safety and tolerability profile as well as significant clinical benefit in last-line tumor patients that were anti-PD-1/-L1 relapsed or refractory. Interim data from the Phase 2 ( NCT04725474 ) trial recently presented at ASCO continue to demonstrate a very good safety and tolerability profile and promising early responses in major cancer indications, including non-small cell lung cancer (NSCLC), bladder cancer and hepatocellular carcinoma (HCC). Mature data readouts for efficacy and safety data of the core Phase 2a program as well as main biomarker-correlations are expected to become available by late 2023.
About the GDFATHER-2 Trials
The GDFATHER-2a trial ( GDF -15 A ntibody-media T ed H uman E ffector Cell R elocation Phase 2) ( NCT04725474 ) is an ongoing Phase 2a trial with several cohorts investigating the effect of visugromab (CTL-002) as monotherapy and/or in combination with a PD-1 checkpoint inhibitor in patients in various advanced-stage, relapse/refractory solid tumor types and a biomarker-selected cohort. The study can enroll > 200 patients in Simon-2-stage designs and a biomarker-evaluation directed cohort to confirm certain response rates and potential biomarker-based responder patient selection.
About Visugromab (CTL-002)
Visugromab is a humanized monoclonal antibody that neutralizes the tumor-derived Growth Differentiation Factor-15 (GDF-15). GDF-15 is an essential player in feto-maternal tolerance, a powerful mechanism that cancer cells hijack to create an immunosuppressive environment to evade destruction. By neutralizing GDF-15, visugromab reverses the immunosuppressive effects that block an efficient anti-tumor immune response in the tumor microenvironment and the draining lymph nodes. Visugromab drives an activated and differentiated immune cell infiltration into the solid tumor as well as enables priming of T cells and enhances the tumor-killing effects of T cells and NK cells. GDF-15 is currently investigated in an ongoing Phase 2 program that includes confirmatory studies in multiple solid tumor indications and the analysis of a predictive response biomarker to better identify the patients benefiting from this new class of immunotherapy.
About CatalYm
CatalYm is pioneering a novel immuno-oncology therapy that safely overcomes GDF-15-mediated immunosuppression in the tumor microenvironment. Our lead product, visugromab, a first-in-class GDF-15 neutralizing antibody, has demonstrated potent and durable anti-tumor efficacy with multiple, long-lasting objective responses in combination with anti-PD-1 treatment in phase 1/2 studies in advanced cancer patients. CatalYm is focused on maximizing visugromab’s potential as a new class of cancer immunotherapy with a clinically distinct profile in a range of solid tumor indications.
View source version on businesswire.com:https://www.businesswire.com/news/home/20230720231828/en/
CONTACT: CatalYm GmbH
Dr. Phil L’Huilier, CEO
info@catalym.com
Media Inquiries
Trophic Communications
Dr. Alison Opalko
Phone: +49 151 54041130
catalym@trophic.eu
KEYWORD: GERMANY EUROPE
INDUSTRY KEYWORD: ONCOLOGY HEALTH CLINICAL TRIALS RESEARCH SCIENCE PHARMACEUTICAL BIOTECHNOLOGY
SOURCE: CatalYm
Copyright Business Wire 2023.
PUB: 07/20/2023 05:00 AM/DISC: 07/20/2023 05:01 AM
http://www.businesswire.com/news/home/20230720231828/en